The Difference Between Life and Living
Sancuso for Chemotherapy-Induced Nausea & Vomiting1
Sancuso helps prevent chemotherapy-induced nausea and vomiting (CINV) so you can focus on what matters most
Understanding CINV and Sancuso:
How CINV Occurs
Why do some patients with cancer experience chemotherapy-induced nausea and vomiting (CINV)?
Chemotherapy has helped millions of patients in their battle against cancer as it destroys cancer cells. Unfortunately, healthy, noncancerous cells are sometimes destroyed too, which can lead to unpleasant side effects, such as CINV.
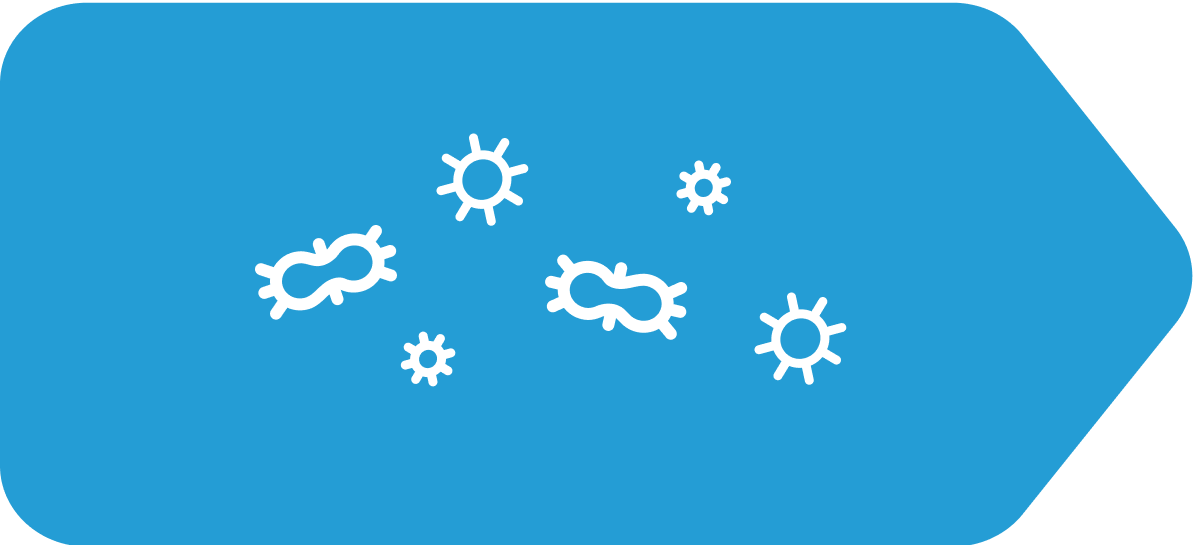
Some chemotherapy may irritate cells in the small intestine and/or the brain
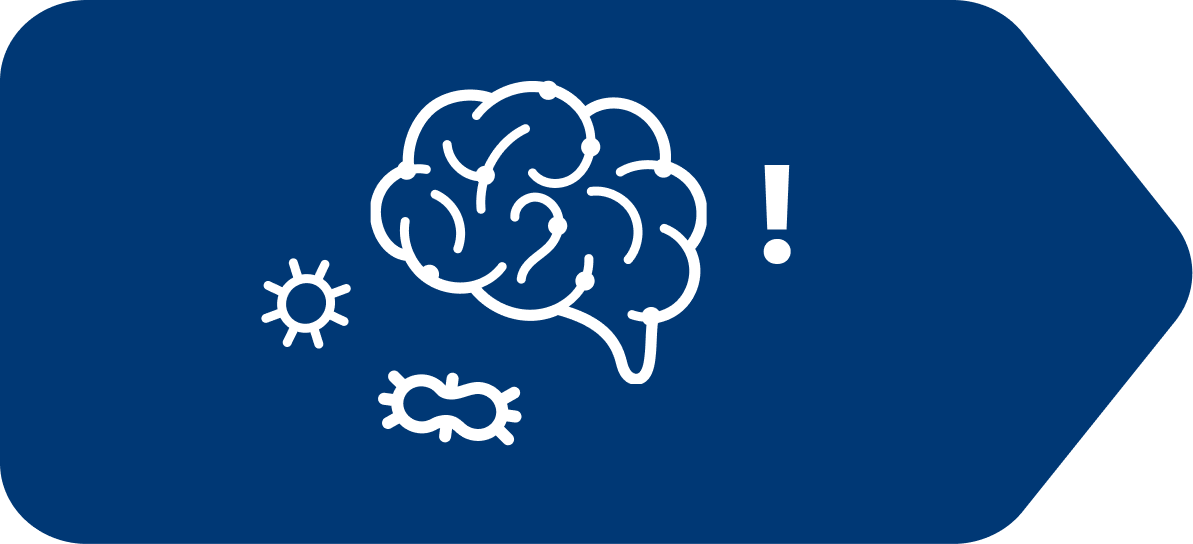
Once irritated, cells in the small intestine send messages to the brain
Upon receiving these messages, the brain instructs the body to react through feelings of nausea and/or vomiting
Risks for CINV2
Some chemotherapy options having up to a 90% chance of CINV occurring:
- AC (doxorubicin or epirubicin with cyclophosphamide)
- Carmustine >250 mg/m
- Cisplatin
- Cyclophosphamide>1500 mg/m
- Dacarbazine
- Mechlorethamine
- Streptozotocin
- Carboplatin AUC >4
- Doxorubicin >60 mg/m
- Epirubicin >90 mg/m
- Ifosfamide >2 g/m per dose

Is Sancuso Right for Me?
If you have chemotherapy complications that may make it hard for you to take oral medications, you may be interested in a different way to prevent CINV
Ask your healthcare provider about a CINV treatment option that may be right for you if you experience any of the following:
- Mouth problems
- Difficulty swallowing
- Gastrointestinal issues

Some patients treated for cancer experience difficulty swallowing pills, feel nauseated, and are unable to keep pills down. This can be caused by certain cancers or certain cancer treatments that may make it hard for you to swallow medicine that prevents CINV.

Some cancer treatments may also reduce the ability of your intestines to absorb or retain the medicine in pills or tablets.

Because Sancuso is a skin patch that enters your system through your skin rather than an oral medication, it might be the right choice for patients who have trouble swallowing or keeping down pills, and those with the reduced ability to absorb or retain medicine in their intestines.
How Does Sancuso Help with CINV?
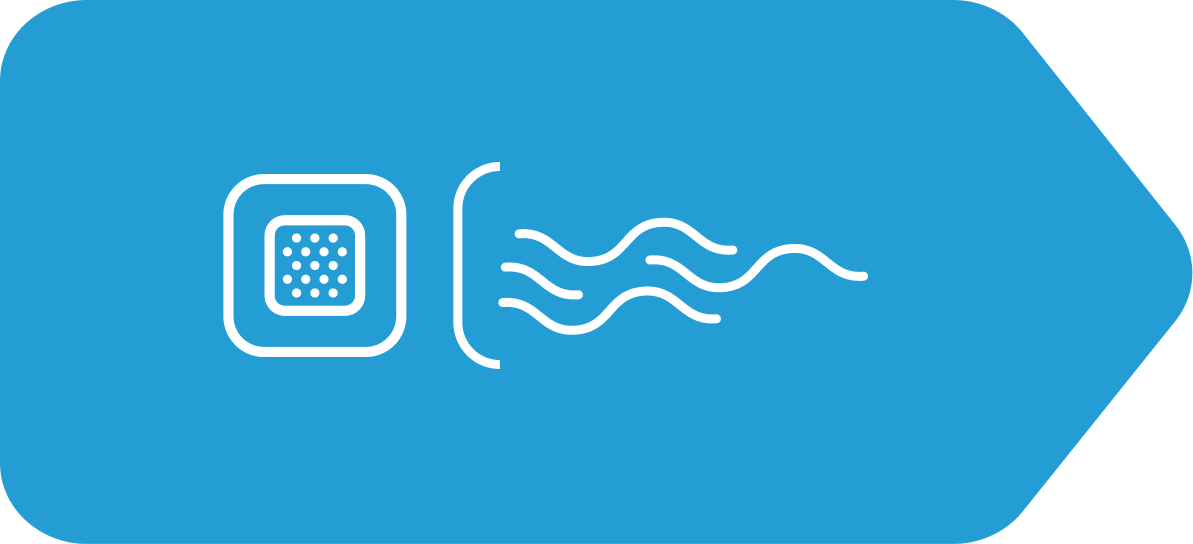
From the time you apply the patch, Sancuso slowly continuously delivers granisetron through your skin and into your bloodstream until the patch is removed within 7 days

Gransitron block cells in the small intestine from sending messages to the brain that triggers CINV
Because the brain does not receive these messages, the feeling of nausea and vomiting are less likely to occur
Sancuso Patient Resources

Co-Pay Assistance
Eligible patients may save up to $300 per patch on Sancuso after paying the first $20 per prescription. Maximum savings of $1200 per month for 4 or more patches.

Side Effects and Safety
Be sure to follow all application instructions carefully and consult your healthcare provider if you have any questions or concerns.
Applying the Sancuso Patch: A Step-by-Step Guide
Simple application for CINV protection
Step 1
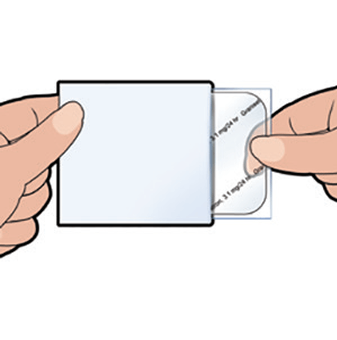
Remove the patch from the carton; tear the pouch open and remove the patch. Each pouch contains one Sancuso patch stuck into a rigid plastic film, and a separate clear protective liner.
Do not remove the Sancuso patch from the pouch until you are ready to use it.
Step 2
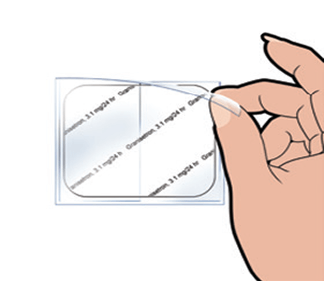
Remove the thin, clear protective liner to expose the printed side of the patch.
Step 3
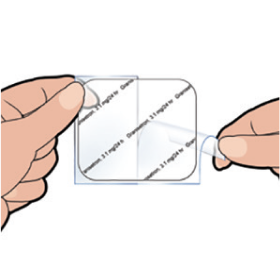
Bend the patch in the middle and remove one half of the rigid plastic film. Be careful not to stick the patch to itself and avoid touching the sticky side of the patch.
Step 4
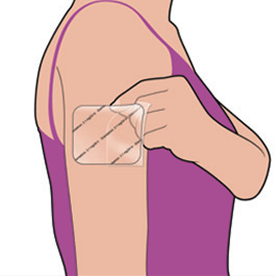
While holding the remaining half of the rigid plastic film, apply the patch to the skin. Remove the second half of the rigid plastic film and press the whole patch firmly in place with your fingers and smooth down. Press firmly, making sure it sticks well, especially around the edges.
Step 5
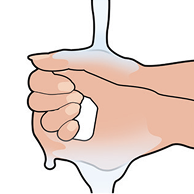
Wash your hands right away after applying the patch to remove any medicine that might have stuck to your fingers.
Step 6
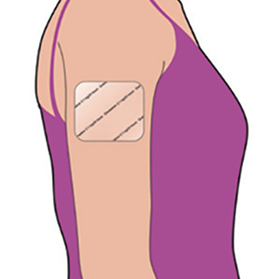
Keep the patch in place for the duration of the chemotherapy. Remove the patch at least 1 day (24 hours) after chemotherapy is finished. The patch can be worn up to 7 days, depending on the number of days that your chemotherapy treatment lasts.
Do not reuse the Sancuso patch after you remove it. See instructions included with your Sancuso patch on how to remove and throw away after use.

Sancuso Guidance
- Be sure to keep the Sancuso patch inside the sealed pouch until you are ready to apply it
- Do not cut the patch
- Apply Sancuso a minimum of 24 hours to a maximum of 48 hours before your scheduled chemotherapy treatment
- Apply Sancuso to a clean, dry, nearly hairless area of skin on the outside of your upper arm. Do not put Sancuso on areas that have been treated with creams, oils, lotions, powders, or other skin care products that might keep the patch from sticking on your skin. Sancuso should not be placed on skin that is red, irritated, cut, or scraped
- If the patch doesn't stick well, you may use medical adhesive tape to keep the patch in place. Place tape on the edges of the patch. Do not completely cover the patch with tape, and do not wrap entirely around your arm
- If the patch comes more than halfway off or it becomes damaged, please contact your healthcare provider
- For our Patch Replacement Program, contact Patient Rx Solutions™ Customer Service Support Phone: 615-425-7642
Wearing Sancuso
- Avoid sunlight. The medicine in Sancuso may not work as well and/or may affect your skin if exposed to direct sunlight or the light from sunlamps or tanning beds. While you are wearing Sancuso, you must keep it covered (i.e., under clothing) if there is a risk of exposure to sunlight or sunlamps. Continue to keep the application area covered for 10 days after removing Sancuso
- Showering or bathing will not change the way that Sancuso works—however, you should try to avoid immersing Sancuso in water for long periods of time to prevent the patch from coming off
- Because there is no information on the effect of swimming, strenuous exercise, or use of a sauna or whirlpool on the patch, you should also avoid these activities while wearing Sancuso
When not to use Sancuso
- Avoid use if you are allergic to granisetron or any of the other ingredients in Sancuso, or if you have allergies to medical adhesive tape, adhesive dressings, or other skin patches
- Avoid use if you are pregnant or breastfeeding unless your healthcare professional has told you that you can use it. Be sure to tell your healthcare professional if you are pregnant, if you become pregnant, or plan to become pregnant while using Sancuso, or if you are planning to breastfeed or are breastfeeding
Possible side effects of Sancuso
- Sancuso can cause side effects in some patients. The most common side effects with Sancuso are constipation and headache
- Be sure to notify your healthcare professional if you have pain in your abdomen or your abdomen becomes swollen
- While you are wearing Sancuso, you may see some mild redness at or around the patch application site. If uncomfortable irritation or excessive itchiness occurs, remove the patch, and call your healthcare professional
INDICATIONS AND USAGE
SANCUSO (granisetron transdermal system) is indicated for the prevention of nausea and vomiting in adults receiving moderately and/or highly emetogenic chemotherapy regimens of up to 5 consecutive days.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
SANCUSO is contraindicated in patients with known hypersensitivity to granisetron or to any of the components of the transdermal system.
WARNINGS AND PRECAUTIONS
PROGRESSIVE ILEUS AND GASTRIC DISTENTION: Sancuso may mask a progressive ileus and/or gastric distention. This should be particularly considered before use of Sancuso in patients who have had recent abdominal surgery. Monitor for decreased bowel activity, particularly in patients with risk factors for gastrointestinal obstruction.
SEROTONIN SYNDROME: The development of serotonin syndrome has been reported with 5-HT3 receptor antagonists. Patients should be monitored for the emergence of serotonin syndrome, especially with concomitant use of sancuso and other serotonergic drugs. If symptoms of serotonin syndrome occur, discontinue Sancuso and initiate supportive treatment. Patients should be informed of the increased risk of serotonin syndrome, especially if Sancuso is used concomitantly with other serotonergic drugs.
SKIN REACTIONS: In clinical trials with Sancuso, application site reactions were reported that were generally mild in intensity and did not lead to discontinuation of use. The incidence of reactions was comparable with placebo. If severe reactions, or a generalized skin reaction occur (e.g., allergic rash, including erythematous, macular, papular rash or pruritus), remove the Sancuso transdermal system.
INCREASED DRUG EXPOSURE WITH USE OF EXTERNAL HEAT SOURCES: Prolonged exposure to heat results in increasing plasma concentrations of granisetron during the period of heat exposure. Do not apply a heat pad or heat lamp over or in the vicinity of the Sancuso transdermal system and avoid extended exposure to heat.
PHOTOTOXICITY WITH ULTRAVIOLET LIGHT EXPOSURE: Granisetron may be affected by direct natural or artificial sunlight, including sunlamps. An in vitro study using Chinese hamster ovary cells suggests that granisetron has the potential for photogenotoxicity. To avoid a potential skin reaction, advise patients to cover the application site of the transdermal system with clothing if there is a risk of exposure to direct natural or artificial sunlight throughout the period of wear and for 10 days following its removal.
ADVERSE REACTIONS
The most common adverse reaction (≥ 5%) is constipation.
You are encouraged to report suspected adverse reactions to Cumberland Pharmaceuticals, Inc. at 1-833-Sancuso or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
REFERENCES
- Sancuso [package insert]. Nashville, TN, Cumberland Pharmaceuticals Inc.; 2024.
- Gupta, K, et al. Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Recommendations, and New Trends. Cancer Treatment Res. 2021;26:100278.
Have Questions or Would like to Stay Informed About Sancuso?
We’re Here to Help!
Have Questions or Would like to Stay Informed About Sancuso?
We’re Here to Help!
